
Specifications
- TissueGnostics scanner with 120 slide loader and Zeiss upright microscope
- Fast scanning of whole slides
- Air objectives: 2.5x/0.09, 5x/0.16, 10x/0.45, 20x/0.5, 20x/0.8, 40x*/0.95
- Oil objectives available upon request: 40x/1.3, 60x/1.4
- Up to 7 fluorescent tags, including near IR (Cy7)
- Sensitive back-thinned sCMOS monochrome camera (Hamamatsu ORCA-Fusion BT C15440)
- Paired with StrataQuest workstation (Marlin) for whole-slide analysis
- Tissue and Tissue Microarray (TMA) detection and handling
- 3 in long x 2 in wide slide holders now available for big sections (50 x 50 mm scan area)
*Please use #1.5 coverslips with the the 40x/0.95 air lens
Sample Preparation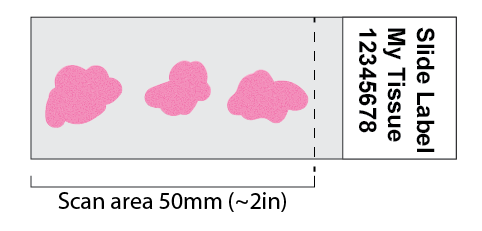
-
Scan area is limited to 50mm from clear edge.
- DO NOT crowd sections together. Spread them out for automated tissue detection.
-
DO NOT use antifade/mounting media with built-in DAPI*. Buy mountant without DAPI and do a separate DAPI stain and wash!!
-
DO NOT use nail polish with a hard-curing mounting media**. Sealing inhibits the curing process.
-
DO NOT freeze your stained slides. Re-freezing slides will destroy your samples. Once stained, store them at 4°C.
-
DO NOT use the following flow-cytometry dyes. Detection (flow) and imaging (microscopy) have different requirements. Some flow dyes and reagents do not work for imaging – especially with multi-band filters. Choose alternatives to these broad-spectrum and other problematic dyes
- ❌❌ PE (Phycoerythrin)
- ❌❌ PerCP
- ❌ APC (Allophycocyanin) - Cy5 or AF647 are preferred
- ❌❌ Quantum Dots
- ❌❌ Brilliant UV/Violet dyes other than BV421
- ❌❌ Primary labeled antibodies in the green channel (FITC, AF488, etc)***
-
DO use one set of fluorescent dyes per slide. Camera/filter scanning settings apply per-slide.
-
DO check the filter list below for recommended fluorescent dyes
-
DO use a #1.5 coverslip over your sample
-
DO use an antifade mounting media (but one without built-in DAPI*)

-
Blank magazine layout templates are available in WORD and PDF formats
*Antifade with built-in DAPI is meant for high-volume clinics. Never buy it for research. The DAPI goes bad very quickly, diffuses into tissues poorly, and always leaves a background signal. Instead, do a separate DAPI stain and wash out the excess before mounting. Why spend hours, days, or months on an experiment and ruin it at the last minute with a poor reagent when a 5-15 minute DAPI stain will work much, much better!
**Hardening mounting medias can take up to 3-4 days to cure. The curing process needs access to air, so do not seal around your coverslip! You can put 4 small drops of nail polish at the corners to hold the coverslip in place while curing. Non-hardening mounting medias are OK to seal. If you do not know which you have, see this Overview of antifade mounting medium page on Fisher for curing vs non-curing mounting media.
***Tissue autofluorescence is strongest in the green channel. It can overwhelm weak primary-labeled antibody signals when imaging. Instead use a polyclonal fluorescent secondary antibody to amplify the signal. There is a similar but weaker autofluorescence in the orange channels (TRITC, AF548). Consider the no-labeled-primary rule for this color too. Remember, flow cytometry and microscopy are different beasts! What works for one doesn't always work for the other.
NEW: The Histology Core Facility has a TiYO Autofluorescence Quencher. After fixation, treat your slides with a peroxide/methanol mix. Then photobleach up to 12 slides for 1-3 hours in a cooling PBS bath. Finally stain your slides. Contact or visit the Histology Core Facility to learn more.
Two Scanning Software Configurations
AllBand (slow scanning) and QuadBand (fast scanning)
AllBand Fluorescent Filter Configuration
Slower scanning using partially separated filters, filters can be mixed-and-matched
See emission spectra comparison
Chroma single band DAPI, low bleed-through, better nuclear separation
| Name | # | Std dyes | Ex | Em |
|---|---|---|---|---|
| DAPI | 5 | DAPI/Hoechst | 390/25 | 460/50 |
Chroma wide-band, bright, up to 5 colors
| Name | #* | Std dyes | Cy series | Alexa | Ex | Em |
|---|---|---|---|---|---|---|
| mDAPI | 6 | DAPI/Hoechst/BV421 | 390/31 | 435/31 | ||
| mFITC | 6 | FITC | Cy2 | AF488 | 479/33 | 519/25 |
| mTRITC** | 6 | TRITC | Cy3 | AF546 | 554/25 | 594/33 |
| mTxRed** | 7 | RFP/TxRed | Cy3.5 | AF594/647 | 578/24 | 641/78 |
| mCy5 | 6 | APC | Cy5/Cy5.5 | AF647 | 637/2 | 695/60 |
| mCy7 | 7 | Cy7 | Cy7 | AF750 | 748/2 | 810/80 |
*# indicates multiband filter cube position. Fluorophores on different cubes have less cross-talk
**These fluorophores are mutually exclusive on the Chroma filters. Use Spectra-Split filters below to image both in the same sample
Spectra-Split narrow-band, better separation, up to 7 colors
| Name | #* | Std dyes | Cy series | Alexa | Opal | Atto | Dylight | Ex | Em |
|---|---|---|---|---|---|---|---|---|---|
| ss380 | 1 | DAPI/Hoechst/BV421 | 390/26 | 424/18 | |||||
| ss440 | 4 | BV480 | Opal p 480 | Atto425 | 435/18 | 469/19 | |||
| ss488 | 1 | FITC | Cy2 | AF488 | Opal 520 | Atto488 | Dylight488 | 491/23 | 525/22 |
| ss546 | 2 | TRITC | Cy3 | AF546 | Opal 570 | Atto542 | Dylight549 | 546/11 | 569/18 |
| ss594 | 3 | TxRed | AF594 | Opal 620 | Atto590 | Dylight594 | 594/13 | 624/30 | |
| ss647 | 4 | APC | Cy5/Cy5.5 | AF647 | Opal 690 | Atto647 | Dylight647 | 637/2 | 686/42 |
| ss750 | 1 | Cy7 | AF750 | Opal p.780 | Atto740 | Dylight750 | 748/2 | 799/50 |
*# indicates multiband filter cube position. Fluorophores on different cubes will have less cross-talk
QuadBand Fluorescent Filter Configuration
Very fast scanning with Chroma multi-band filter, up to 4 colors without filter change delays
| Name | # | Std dyes | Cy series | Alexa | Ex | Em |
|---|---|---|---|---|---|---|
| mDAPI | 6 | DAPI/Hoechst/BV421 | 390 | 435 | ||
| mFITC | 6 | FITC | Cy2 | AF488 | 479 | 519 |
| mTRITC | 6 | TRITC/TxRed | Cy3 | AF546/594 | 554 | 594 |
| mCy5 | 6 | APC | Cy5/Cy5.5 | AF647 | 637 | 695 |
Other Dyes
Here is an Excel Table and PDF version of other dyes sorted by peak emission wavelength. Keep in mind that dyes are shown by their peak wavelengths, but both excitation and emission spectra are usually much broader than shown.
Please see the FPBase spectrum viewer, BD Spectrum Viewer, ThermoFisher Spectrum Viewer, Chroma Spectrum Viewer, or your favorite online source for spectrum.
Objectives and Extended Depth of Focus
Recommended Z-STEP sizes for extended depth-of-focus imaging
| Objective | OIL | Z STEP | Corr | WD mm | Z DOF µm |
|---|---|---|---|---|---|
| 2.5x/0.085 | EC Plan-Neofluar | 8.80 | 106.71 | ||
| 5x/0.16 | EC Plan-Neofluar | 18.50 | 29.61 | ||
| 10x/0.45 | 4.0 µm | Plan Apochromat | 2.00 | 4.16 | |
| 20x/0.5 | 2.5 µm | EC Plan-Neofluar | 2.00 | 2.85 | |
| 20x/0.8 | 1.0 µm | Plan Apochromat | 0.55 | 1.27 | |
| 40x/0.95* | 0.8 µm | Plan-Apochromat Korr | 0.25 | 0.78 | |
| 40x/1.3** | OIL | 0.6 µm | EC Plan-Neofluar | 0.21 | 0.68 |
| 63x/1.4** | OIL | 0.5 µm | Plan Apochromat | 0.19 | 0.54 |
*The 40x/0.95 air objective requires additional training
**Limited availability. See staff for OIL objective use
Data Files and Software
Acquired Data Files
- The current acquisition version (7.xxx) uses a proprietary format, but with a free viewer for export to ome-tiff
- The next acquisition version (8.xxx) stores slides as single files. A native bio-formats reader is coming soon. Until then, the 8.xxx viewing software can export as ome-tiff.
- Acquired slide files include the small index file and ALL the subdirectories below them.
- Jobs are indexed by "jobname.bfproj" and are a collection of 1 slide projects
- Slides (1 slide projects) are indexed by "slidename.aqproj"
- Keep a "golden master" of your data as it was acquired, not just the exported files.
- Make sure to back up everything, not just the tiny .bfproj and .aqproj files.
Viewing/Export Software
- TissueFAXS Viewer software is available for download here [StrataQuest link].
- Make sure to download the version for "Systems with Slideloader".
- It should have "SL" in the name
- The viewer only runs in Microsoft Windows
- The viewer can export the stitched image into ome-tiff format
- The viewer also includes a command-line batch exporter